CPChain and Drug Safety: Smart Healthcare Solutions
by CPChain at Aug. 9, 2018
Throughout the past decade, China has faced several acts of unethical behavior regarding drug safety. The Jilin Changchun vaccine scandal is a recent example in which the company was accused of falsifying data for a rabies vaccine and manufacturing an ineffective vaccine for babies. Other examples include the illegal vaccine scandals in Shandong in 2016, Zhejiang in 2014 and 2011, and Hubei in 2010.
The Chinese government has issued a series of policies and regulation in order to deal with the ongoing safety concerns. One of these strategies is the electronic code of drugs, introduced in 2008, which collaborates information technology, network technology, and coding technology in order to develop an electronic surveillance code for drugs. Enterprises were required to upload information through the electronic regulatory system so that drugs can be identified and monitored in real-time.
While the electronic code of drugs played a vital role in combating counterfeiting and inferiority drugs, issues were gradually raised and the system was terminated by the government in the year of 2016.
Currently there are no valid drug supervision systems in both national and organizational levels. With the increasingly dangerous drug safety scandals being reported, the public is demanding valid and practical solutions for the protection of public health and safety.
Pharmaceutical Supply Chain
The pharmaceutical supply chain is an overall functional network chain including suppliers, manufacturers, distributors, and end-users. Below is a basic workflow diagram of a pharmaceutical supply chain:
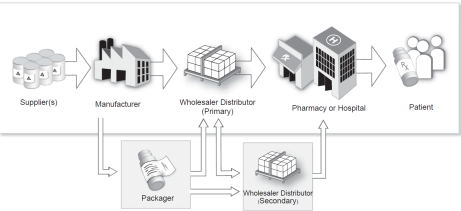
Basic workflow diagram of a pharmaceutical supply chain
Pharmaceutical suppliers provide raw materials for manufacturers who then start production. After the various steps during the production process, the finished products are shipped to the next-level regional distributors. Prior to entering the distributors, the drugs need to be properly repackaged for ease of transportation and sales. After passing through a multi-level distributor, the drug flows to the terminal to sell to medical institutions such as pharmacies and hospitals. At the end of the process, the consumers purchase the drugs from these medical institutions.
Through this pharmaceutical workflow, we can reasonably conclude that drug safety issues can occur at any stage. Furthermore, we must take into account that the danger of drug scandals has grown exponentially at the raw materials stage.
Blockchain Application Potential
Blockchain technology, i.e., distributed ledger technology, is a combination of P2P networks, consensus algorithms, cryptography, and databases. Blockchain focuses on the following three issues in the field of drug supply chain traceability and supervision:
- Trust Refactoring — through the consensus agreement for the traceability of the pharmaceutical industry, businesses can conduct legal transactions and the appropriate consensus algorithm is adopted to realize the trusted transaction involving multiple parties in the supply chain.
- Privacy Protection — through the encryption mechanism, the privacy information of the business is guaranteed without affecting the supply chain workflow.
- System Security — through the P2P network architecture of Blockchain, the system security has improved stability and anti-aggression compared to a centralized server mode.
The Blockchain Solution
CPChain (Cyber-Physical Chain) is a new generation of IoT distributed infrastructure, which combines distributed storage, data encryption computing, and Blockchain technology. CPChain is reshaping the basic architecture of the Internet of Things and building an IoT-oriented network. The system’s basic data platform provides a full-process solution for data acquisition, storage, sharing, and application. CPChain solves a series of challenges in the existing “chimney” system of the Internet of Things, reduces equipment interconnection costs, and effectively protects data privacy.
System Structure
After examining the many pain points in the field of drug supply chain traceability and supervision, CPChain has proposed the following Blockchain-based system structure:
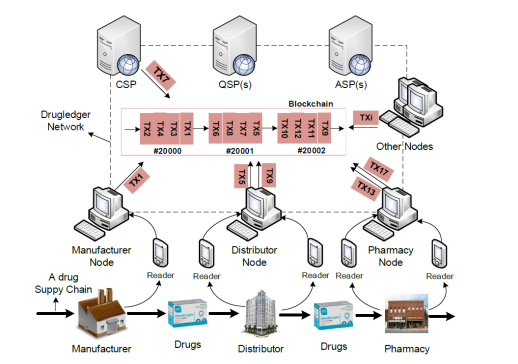
Blockchain-based system structure for drug supply chain traceability
As shown in the illustration above, the service role of the traditional traceability system is divided into three parts in the system, a Certificate Service Provider (CSP), a Query Service Provider (QSP), and an Anti-System Attack Service Provider (ASP).
The Certificate Service Provider (CSP) mainly provides a Blockchain access mechanism which requires Blockchain participants to provide legal certificate credentials. This effectively prevents against witch attacks where in a peer-to-peer network, a single node with multiple identity identities weakens the role of redundant backups by controlling most of the nodes of the system. In general, a large number of falsified identities exist in the network, and malicious activities are performed so that the system cannot work normally. On the other hand, the Blockchain system also prohibits unapproved drug manufacturers from participating in chain transactions. In reality, the certificate service provider is the Drug Administration (CFDA).
A query service provider (QSP) can be any node on the Blockchain system that provides query and retrieval of drug information flows primarily for Blockchain participants and end consumers. Blockchain users can choose to register the relevant QSPs.
Anti-Attack Service Provider (ASP) plays the role of “anti-virus software” in the drug traceability and supervision system to detect malicious behaviors that may occur in the system. For example, to detect whether participants have abused transactions. As the system is updated, the anti-attack service provider itself can continuously adapt to the periodic iteration of the system.
Basic Workflow
With full consideration of the information physics characteristics of the drug supply chain traceability system, we propose an extended UTXO model (UTXO is called Unspent Transaction Output, which means unconsumed transaction output. Unspent transaction output UTXO is a containment transaction) The data structure of the data and execution code can be understood as a cryptographic digital currency that has been received by an address but has not yet been spent. Blockchain-based cryptocurrency uses UTXO to verify that an address has unused encrypted digits. Money is used to pay for the drug supply chain workflow.
At the data model level, a number of transaction types for adaptation scenarios have been redefined, such as: production, drug leaving, reaching new arrival, package, etc. Specifically:
The arrival and departure of drugs is performed locally by the relevant parties in the drug supply chain, and the basic transaction logic is completed; when the drug is first produced in the smallest package, the manufacturer performs passive drug transactions; when a drug transaction needs to be cancelled when the time (such as the other party’s private key is lost), the relevant manufacturer can cancel the transaction, and the offline operation corresponding to the operation (or re-operation, or cancel operation).
Packaging Issues
Different levels of packaging on the drug supply chain are an important part of implementing the drug traceability function on the Blockchain system. The chart below shows the distribution process for drugs at different packaging levels in the pharmaceutical supply chain:
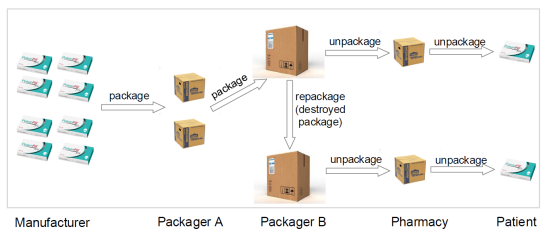
Distribution process at different packaging levels in the pharmaceutical supply chain
This different level of packaging is difficult to directly make meaningful associations because it is identified by an encrypted hash on the chain. Our solution is to make the package itself an effective association as part of a chain transaction. Implement specific packaging associations for different packaging scenarios based on the improved UTXO data model:
First of all, in order to reduce the level of packaging and high-level packaging, we will use the UTXO corresponding to the current low-level packaging as the transaction input, perform a blockchain transaction, and realize the “packaging” of the drug after completing the relevant transaction legality verification.
Secondly, considering the case where the packaging damage needs to be repackaged, on the basis of the above packaging transaction, the legality of the relevant transaction is detected, and the packaging is completed again, and the repackaging is completed.
Finally, for the packaging disassembly process of the drug in the distribution stage, the unpacking transaction type is defined, and the package disassembly is completed.
Storage Issues
Increasing storage is an important issue in Blockchain systems. It is difficult to reduce the storage capacity from the perspective of the general Blockchain. The main problem is how to reduce the system storage space without affecting the normal operation of the system. In contrast, we propose a pruning method based on the drug life cycle from the actual scene of the specific drug supply chain.
Due to regulatory reasons, most of the circulating drug life cycles in the market generally do not exceed 5 years, so there is an effective cycle of drug trading in the Blockchain system. Further, if the linear block organization mode is adopted, the block life cycle can be controlled within a certain range, so that the overall storage capacity of the drug chain traceability system based on the Blockchain can be maintained for a certain period of time to achieve the final stable storage.
Smart Healthcare Solutions
The traceability and supervision of the pharmaceutical supply chain is a complicated issue that requires both strong policy support and effective technological supports. Based on our research, we believe that the Blockchain has great potential in the field of pharmaceutical supply chain traceability and supervision. CPChain will play a vital role in the field of smart healthcare and Blockchain applications related to drug safety and supply chain traceability within China.





